Cancer is one of the main causes of death worldwide with almost 10 million deaths occurred in 2020 [1]. Glioblastoma is a devastating cancer of the brain whose cancer cell proliferation is very fast and aggressive. To study, treat and destroy brain tumor cells, researchers are investigating proton beam radiation which has proven to be a more effective and minimal invasive technique than x-ray radiotherapy in different cancer types. However, the costs of proton beam therapy are high, which makes the testing in animals and humans also very costly and almost inaccessible. The higher costs in proton radiotherapy also lead to a lack of clinical studies to understand the effects of protons on glioblastoma at the cellular level.
Biomimetic cellular scaffolds by 3D printing
Scientists at the Delft University of Technology led by Professor Angelo Accardo tackle the challenge with a promising novel approach to study in vitro glioblastoma cell cultures under proton beam radiation, featuring the cover of ACS Applied Materials & Interfaces. They develop in vitro cell cultures made of 3D engineered cellular microenvironments. For this purpose, they use Two-Photon Polymerization (2PP) to fabricate biomimetic 3D scaffolds that mimic the geometry of brain blood vessels. The biomimetic geometry affects the glioblastoma cells and their colonization mechanics. In these experiments, the 3D cell culture is scaffold-based and thus cells can grow in a controlled manner guided by a scaffold geometry in 3D. The very confined photopolymerization by 2PP occurring only in the tight laser focus allows to 3D-print finest features in the submicrometer range. Moreover, this additive manufacturing technology exploits the full 3D design freedom at the micrometer scale and is relevant for mimicking 3D cellular microenvironments with the highest precision.
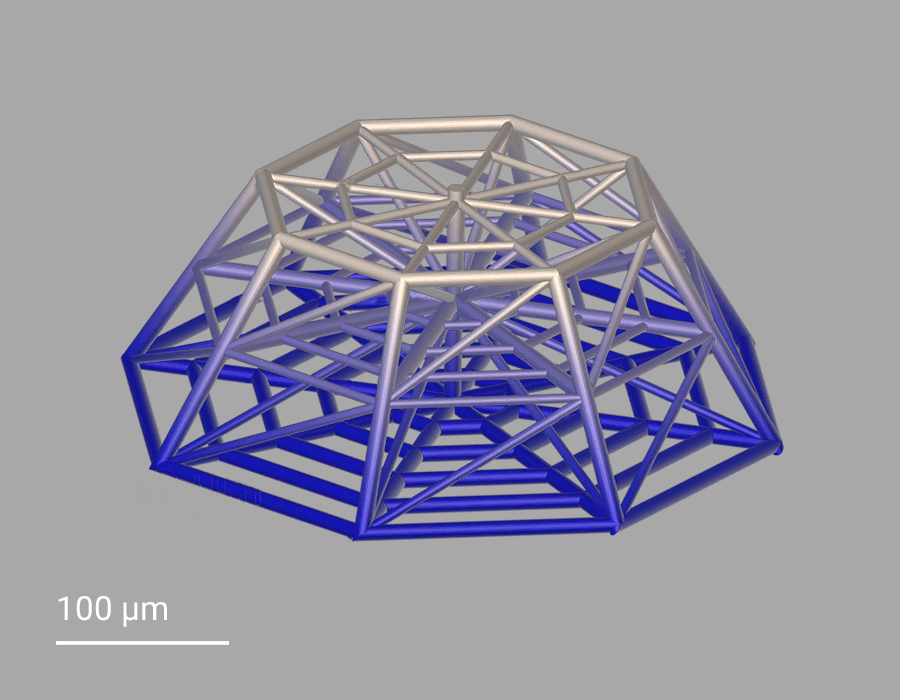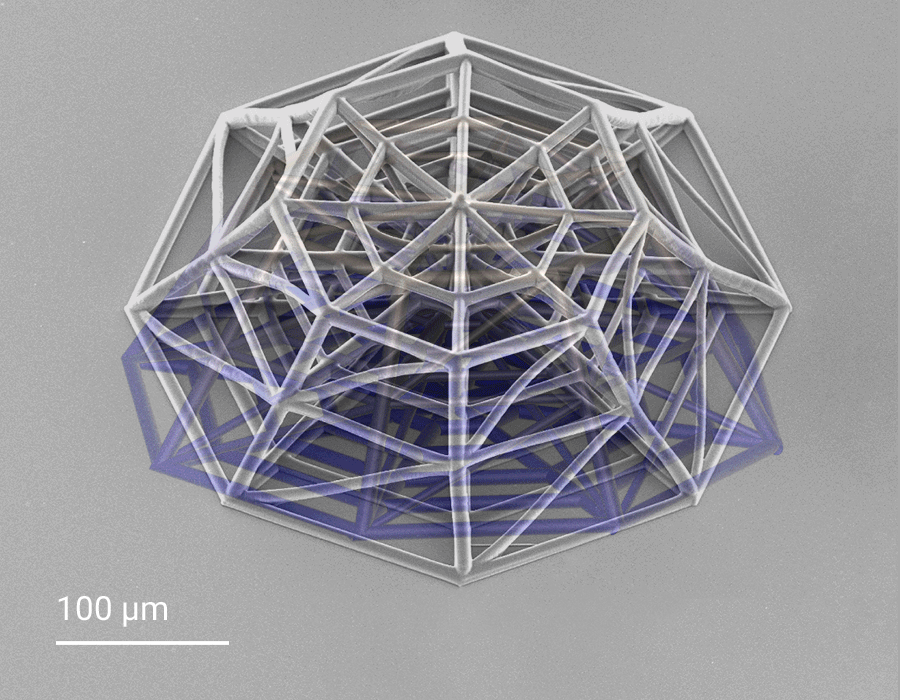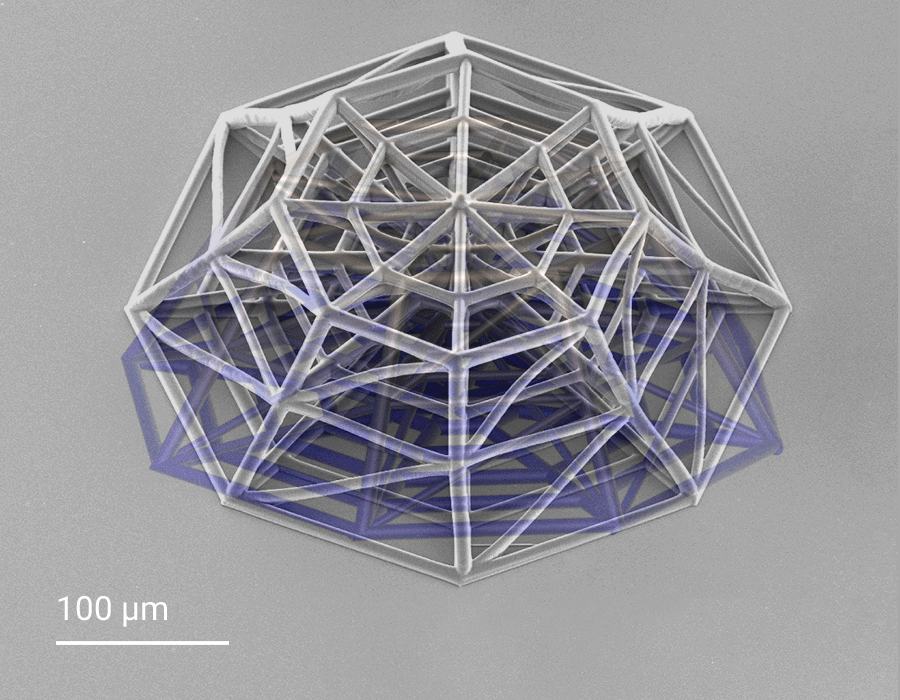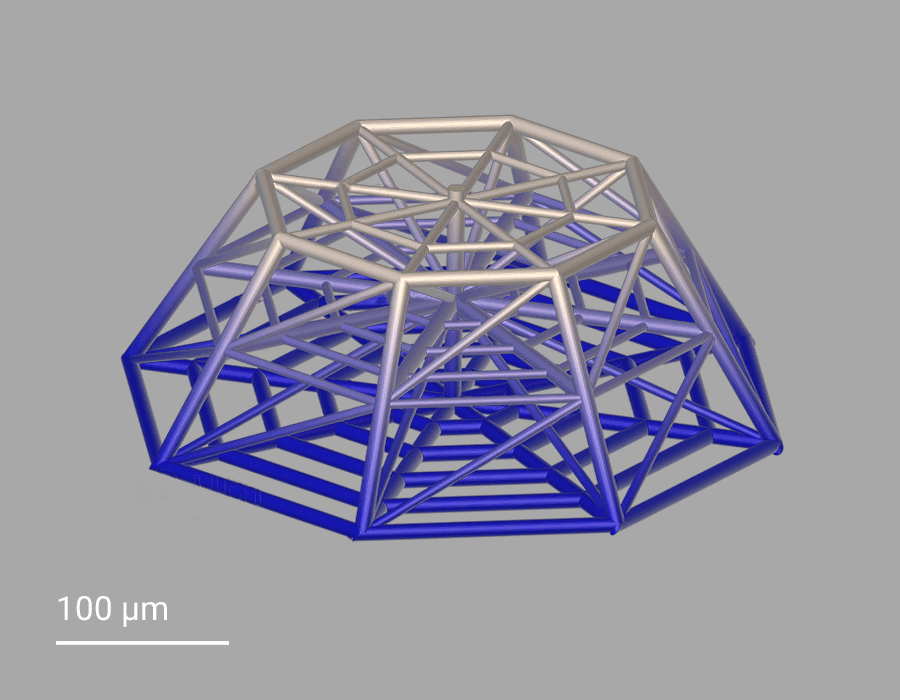
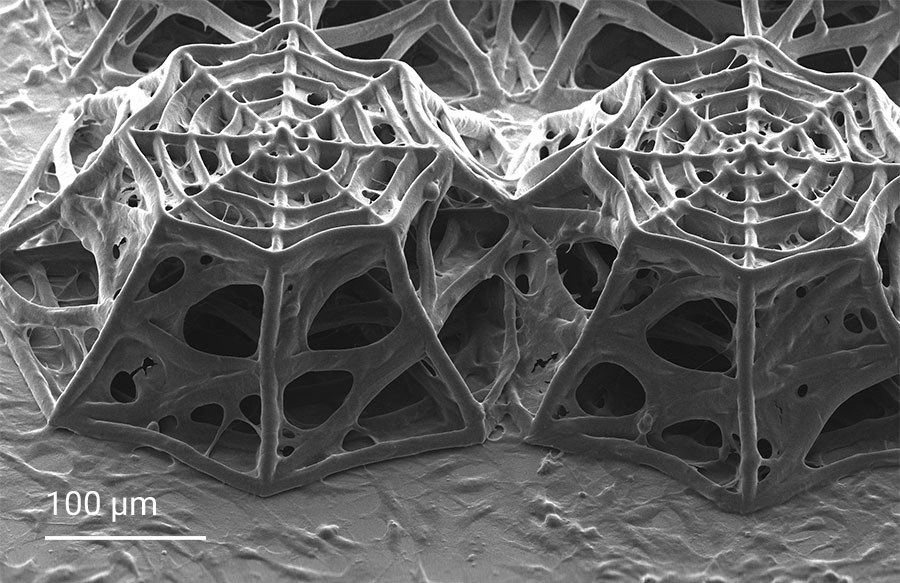
In this project, cellular scaffolds with only 150 micrometers height are 3D-printed. The scaffolds include an octagonal base and a top octagonal layer similar to the bottom one in a smaller size, forming octagonal truncated pyramids. Confocal microscopy benefits from the truncated pyramids that allow analyzing a quantitatively large amount of cells, while SEM images show more details on the cell morphologies associated with the geometry of the scaffolds. In the 3D microenvironment, the cells often adopt a more spherical form than on 2D pedestals. The pyramids include horizontal and inclined beams, with diameters of 10 and 6 µm, that meet pretty well the cellular scale. The beams provide regions for cell adhesion and mechanical stability to avoid the scaffold to collapse under cellular mechanical stimulation. The 3D cellular culture is characterized by the cytoskeleton that is extended from the cells in the directions of the beams of the scaffold. While on 2D pedestals the cells form unrealistic cellular flat monolayers, the cells on the 3D microenvironments colonize the inner core of the scaffolds as well as the outer framework of the architecture.
Dedicated biomaterial for cell culture, growth, adhesion and microscopy
The scaffolds are made of Nanoscribe’s IP-Visio photoresin, which is a non-cytotoxic material according to ISO-10993-5. This biomaterial is beneficial for cell biocompatibility, growth and adhesion of glioblastoma cells. Furthermore, IP-Visio has a negligible autofluorescence that does not interfere with cell staining when used for cell microscopy. Thus, IP-Visio is suitable for immunofluorescence imaging of cell biomarkers. For the use of IP-Visio in this particular cell application, there was no need for additional biofunctionalization or biochemical coating, as the material promoted the required cell adhesion on the structures.
Moreover, IP-Visio’s Young’s modulus measured on 2D pedestals is 1.31 GPa, almost 50 times lower than the Young’s modulus of conventional soda-lime glass substrates (E ≈ 70 GPa) that are typically used for cell cultures. Although it is still far from the Young’s modulus of the brain extracellular matrix, IP-Visio provides a rather soft environment that stimulates cell-cell and cell-scaffold interactions and very clearly different cellular morphologies on 2D and 3D microenvironments. Thus, the combination of Nanoscribe’s Two-Photon Polymerization and IP-Visio paves the path to engineer intricate biomimetic environments made of a cell-friendly biomaterial that can provide mechanical, biochemical and geometric stimuli to cells.
Exploring cancer cell behavior in proton therapy
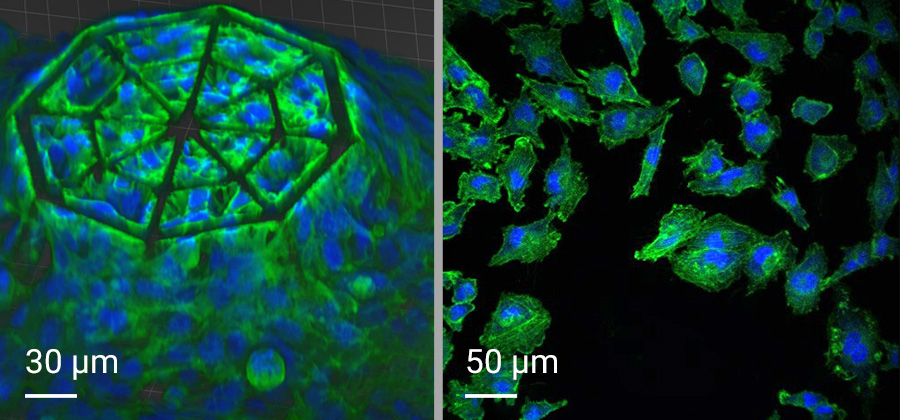
Using the capabilities of high-precision 3D printing and a suitable biomaterial, 3D-engineered scaffolds are fabricated and used as glioblastoma microenvironments. After sterilization of the scaffolds, glioblastoma cells are cultured on the scaffolds for 5 days. The same procedure is repeated for 2D pedestals for comparison purposes. After this time, the cells-colonized scaffolds undergo exposure to proton beam radiation. Upon proton irradiation, confocal microscopy was used to analyze the cells on the 2D pedestals and 3D microenvironments before and after proton radiation. In the confocal images, a fluorescent biomarker (Gamma H2A.X) revealed the formation of a higher number of DNA damage foci in cells on 2D pedestals compared to 3D microenvironments. The number of foci is related to the DNA damage in a cell, confirming a lower DNA damage of glioblastoma cells in 3D cell cultures than in 2D cell monolayers, which correlates with the response of glioblastoma cells in vivo.